Environmental Health and Safety Blog | EHSWire
The 2012 TSCA CDR Submission Period Begins! Rev up your calculators and keyboards, NOW!!
Posted by Shivi Kakar
Topics: Emilcott, TSCA & R.E.A.C.H., TSCA, CDX registration, CDR, Toxic Substance Control Act
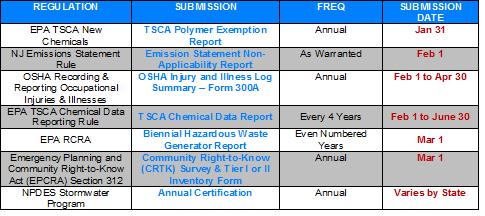
Regulatory Submissions & Postings Reminder (January thru April 2012)
Posted by Shivi Kakar
Topics: Emilcott, NPDES, OSHA, Emergency Planning and Community Right-to-Know Act, General Industry H&S, OSHA Compliance, General EHS, Construction H&S, EPA, EPCRA, Hazardous Waste Management, TSCA & R.E.A.C.H., TSCA, CDR, Toxic Substance Control Act, Biennial Hazardous Waste
At Emilcott, 2011 was a successful year where we were able to tackle new projects, serve our clients and continue to respond to current industry issues in the environmental, health and safety field. Though we have had many great memories from 2011, we would like to mention some of the things that stood out most in our business for 2011.
Hurricane Irene the immense and powerful Atlantic hurricane that left a path of destruction and devastation was something that definitely stands out in 2011. We remember this event not only because of the wreckage caused by the storm, but also because of the after effects. The staff at Emilcott recognized the importance of addressing the legacy of water intrusion and the promise of mold after the storm and stressed to clients the importance of timing to address how to respond to this problem, as well as the importance of selecting the proper remediation technique along with an EHS mold expert and Emilcotts mold remediation strategy. (Read more: Hurricane Irene Leaves a Legacy of Water Intrusion and the Promise of Mold)
Energy Sector Emilcott has been thrilled to be able to participate in many different initiatives within the energy sector. Regionally important to the growth of our economy, the ongoing infrastructure improvements have given us substantial health and safety support work. In EHSWire during 2011 we addressed many occupational hazards as Occupational Heat-related Illnesses where we went over the symptoms that workers may experience, as well as what should be done if someone does experience these symptoms. Besides dealing with working conditions such as heat, Emilcott also provided information on the truths about occupational slips, trips and falls which ended up costing American businesses $13.67 billion in workers compensation costs in 2008. Adhering to proper safety protocols and preventing injuries is something that benefits businesses and their workers. OSHA provides a Walking/Working Surfaces Safety and Health Topic page which provides links to all the applicable standards.
With issues such as heat affecting the health of workers to preventing injuries on job sites, Emilcott has seen our fair share of mishaps. Being able to share our experiences and knowledge with others never gets old. From teaching someone the hazards about working near a crane, or things you should do when working in certain environments, Emilcott has always tried keeping people in the loop. We even have a 10-Hour Construction Industry Outreach Training Course based on the requirements established by OSHA which is a very hands-on and interactive class that we recommend to avoid a future work related issue. (Read more: Work Near a CRANE? Learn the Hazards!)
9/11 Tenth Anniversary focused the changes that have occurred since 9/11/2001 such as the new precautions that have taken place on the American Chemical Security issue. The DHS (the Department of Homeland Security) has been increasing their focus on utilities and chemical facilities which may become targets for terrorist activities and the DHS Chemical Facility Anti-Terrorism Standard (CFATS) now requires completing and submitting a Top Screen analysis to the DHS.
The James Zadroga Act , which was authorized to broaden, renew funding and extend benefits to Ground Zero workers whose death was a result of exposure, is of great significance and has put new emphasis on the importance of proper real-time environmental site monitoring. New technologies are available to protect site workers and the public from exposure to hazardous substances such as those from the collapse of the WTC towers. (Read more: 9/11 Tenth Anniversary Focuses on American Chemical Security)
Toxic Substance Control Act (TSCA) was of major importance not only to Emilcott, but also to facilities who are manufacturers or importers of chemicals in amounts of 25,000 pounds or greater. With so many questions regarding TSCA and the changes, Emilcott decided to put on a free webinar along with posting a number of blogs that answered many of the concerns our clients had. Emilcott was able to use its expertise and help many clients with TSCA compliance questions and concerns regarding the developments of IUR reporting and reporting obligations in 2011 for the calendar year 2010. (Read more: August 2011 Update on the TSCA IUR-now-CDR Rule)
Though Emilcott has had many remarkable memories of 2011, we felt these 4 really left an impression on our business. Emilcott is privileged to know that we were able to assist our clients in many different businesses not only in 2011, but throughout our history. Emilcott looks forward to a productive 2012 and we are excited to see what this year has in store for us.
Do you have any environmental, health or safety concerns for 2012? If so, please share them with us below!
Topics: Emilcott, OSHA, health and safety, CFATS, Hazardous Materials, worker safety, Occupational Health, Occupational Safety, TSCA, Toxic Substance Control Act, Uncategorized, Mold
Emilcott TSCA Resource Center Expands with More Info and Options
Posted by Shivi Kakar
TSCA questions are pouring in and we are responding. To ensure that the information is available in a reasonable (and non-overwhelming) way, Emilcott has created a TSCA resource section of our web site for centralizing all kinds of intelligence, notifications, links, and summaries about Toxic Substance Control Act (TSCA) 2012 Chemical Data Reporting (CDR).
All these pages (just click on the headers) are available from the Emilcott Home page but we suggest you bookmark the pages that are most relevant and dont forget to register for our December 6th Free Webinar!
Emilcott TSCA Resource Center
The TSCA Resource Center has moved from the Emilcott home page to a new page lots of information including all our TSCA-related EHSwire blogs (CDR and IUR) and EPA Chemical Data Reporting Links, EPA New Chemicals Links, and EPA Import/Export Links. This page will stay updated so that you have a one-stop location for all TSCA information. If you have specific issues youd like to see addressed here, please let us know.
Topics: Compliance, TSCA & R.E.A.C.H., TSCA, CDR, Toxic Substance Control Act, IUR, violation, regulations, questions, filing, reporting, Chemical Data Reporting, Form U
EPA TSCA Regulatory Update: A Preview of the CDR Form U Submission Tool
Posted by Shivi Kakar
If you are a foreign or domestic business in the US who is either a chemical importer (resells for use in blending, repackaging) or chemical manufacturer (make new chemicals out of chemicals purchased from others with the exception for pharmaceutical companies), this update is about mandatory compliance with the EPAs Toxic Substances Control Act (TSCA), specifically filing the 2012 TSCA Form U Chemical Data Report.
Emilcott recently participated in an industry preview (the unveiling!) of the e-CDRweb tool. Based on this peek, we are optimistic that the tool will be functional and surmountable IF all the required information is gathered together prior to preparing the submission. As a test run, we entered simulated data and found the online tool to be logical and the built-in validation system should assist submitters with identifying inconsistent or incomplete entries.
Our conclusion: The difficulty will most likely not be the use of the e-CDRweb tool, the greater challenge will be the effort and time required to gather the right data needed for the submission.
Our advice: START GATHERING THE REQUIRED DATA NOW!!
Start with the following 2010 and 2011 inventory and volume data:
- Review the Form U data needed, consider the time you will need to obtain these data, and then allow additional time for getting follow-up, incomplete or missing information.
- Determine Co-Submitters for chemicals that are toll manufactured.
- Get the CAS number for all chemicals at or above the 25,000 lb threshold. Prescient warning: the need to submit a CAS number or accession number for each chemical may require significant effort and time for submitters with suppliers that list confidential for the component.
- Define which suppliers will need to be joint submitters.
- Discuss and agree upon this with the supplier.
Get the CDX registration and authorizations completed a multi-step, multi-party and possibly lengthy process.
- CDX registration for e-CDRweb will be available on November 1, 2011.
- Primary Authorized Official must be registered first as this activates the account.
- Designate the Primary Support
- Establish the Secondary Authorized Officials (joint submitters) by chemical substance
In summary, if you were to compare filing the EPAs TSCA submission to the IRSs income tax form, the e-CDRweb tool is definitely going to be easier. However, understanding what to get and where to get it and then digging up the required information for the TSCA submission is going to be challenge.
If you need guidance with what data you should for the EPA TSCA 2012 CDR Submission, please contact Emilcott!
You can also subscribe to our free TSCA e-newsletter which delivers information right to your mailbox. Want more info? Enroll for our free Dec webinar by sending an email to pkaufmann@emilcott.com.
Feel free to post any questions below in the comments section and we will respond quickly.
Topics: EPA, Compliance, TSCA & R.E.A.C.H., TSCA, Toxic Substance Control Act, submission, IUR, cdx, chemical manufacturer, Secondary Authorized Official, chemical data report, regulation, Primary Authorized Official, eCDRweb
What You Need to Know: TSCA 2012 CDR Form U Submission
Posted by Shivi Kakar
If you are a foreign or domestic business in the US who is either a chemical importer (resells for use in blending, repackaging) or chemical manufacturer (make new chemicals out of chemicals purchased from others with the exception for pharmaceutical companies), this update is about mandatory compliance with the EPAs Toxic Substances Control Act (TSCA), specifically filing the 2012 TSCA Form U Chemical Data Report.
Information about the new TSCA CDR Form U reporting tool is rolling in from the EPA. On September 23 rd the Agency hosted an hour-long webinar in which the 2012 CDR reporting requirements were reviewed and the use of the electronic Form U reporting tool (e-CDRweb) was demonstrated. If you missed the webinar or need a rewind, both the presentation materials and recorded webinar have been posted by the EPA at IUR/CDR About Submissions.
Webinar Take Aways
- The Agency is emphasizing two new reporting requirements:
- The standard of known to or reasonably ascertainable by for processing and use information (formerly readily obtainable)
- The upfront Confidential Business Information (CBI) substantiation
- Both the company that contracts for the manufacture AND the toll manufacturer are now considered to be the co-manufacturers of that chemical substance
- The e-CDRweb tool is designed for joint reporting and has very specific requirements for supplier-EPA communication. Joint reporting is specifically for those instances where a supplier will not disclose the specific chemical name (or TSCA accession number) of a chemical substance or a reactant used to manufacture the TSCA chemical substance because the name is claimed confidential.
- Registration with the EPAs Central Data Exchange (CDX) is required prior to accessing e-CDRweb.
- CDX registration requires the completion of an electronic signature agreement (ESA) form that foreign suppliers must submit by mail.
- CDX registration for e-CDRweb will be available on November 1, 2011.
- CDX registration is a multi-part process.
- The EPA will be providing support for use of the new e-CDRweb tool.
- A training webinar is tentatively scheduled for November 2011.
- Comprehensive instructions for the 2012 TSCA Chemical Data Reporting are now online.
To summarize, the e-CDRweb tool looks like it is a much friendlier submittal tool than its predecessor, e-IURweb. The gotcha will be in the preparation of the materials so that the submittal process is easy. Like many other federal filings, understanding what to submit and why may be much more complicated than the actual filing process. The requirements that changed from the 2006 to 2012 range wildly from subtle and minor to extensive and complex.
Did you participate in the EPAs e-CDRweb webinar? What did you think? Are there any particular gotchas that caught your eye?
If you need guidance with what data you should for the EPA TSCA 2012 CDR Submission, please contact Emilcott for help
- You can also subscribe to our free TSCA e-newsletter which delivers TSCA-related information right to your mailbox.
- Want more info? Enroll for our free Dec webinar by sending an email to pkaufmann@emilcott.com.
If you have any questions, feel free to post them in the comments section and we will respond quickly.
Topics: EPA, Compliance, TSCA & R.E.A.C.H., TSCA, Toxic Substance Control Act, submission, IUR, reporting, Form U, cdx, regulation, eCDRweb, central data exchange
Start collecting data for your 2012 TSCA Chemical Data Reporting submission now!
Posted by Shivi Kakar
We just had our first cold weather snap in the northeast reminding me that 2012 is just around the corner. A change in the seasons is our cue to ask Emilcott clients that manufacture or import chemicals: Have you gathered the 2010 chemical volume data AND are you collecting the 2011 data needed for the 2012 TSCA Chemical Data Report?
In a previous blog we summarized the basic requirements of the inventory, production volume and use i nformation that needs to be collected for the EPAs TSCA 2012 Chemical Data Report (CDR). You can find this bare bones chart by clicking here. The EPA recently presented the following key requirements in this document Instructions for the 2012 TSCA Chemical Data Reporting.
HIGHLIGHTS OF 2012 TSCA CHEMICAL DATA REPORTING (CDR)
- The determination of the need to report is based on production volume during calendar year 2011.
- Information on the reportable chemical substance must be reported during the 2012 CDR submission period, February 1, 2012 to June 30, 2012 (40 CFR 711.20).
- All reporting companies must report CDR data electronically, using e-CDRweb, the CDR web-based reporting tool, and EPAs Central Data Exchange (CDX) system. Prior to submitting data, submitters must register with CDX.
- Reporting is required for all chemical substances listed on the TSCA Inventory, both organic and inorganic, other than polymers, microorganisms, naturally occurring chemical substances, certain forms of natural gas, and water (40 CFR 711.5 and 711.6) when manufacture (including import) of those chemical substances meets the other reporting requirements. Chemical substances that are the subject of any of certain listed TSCA actions may not be eligible for partial or full exemptions (40 CFR 711.6).
- Manufacturers (including importers) are required to report full manufacturing data, for calendar year 2011, and production volume only, for calendar year 2010, for all reportable chemical substances, when 2011 site-specific production volume equals or exceeds 25,000 lb (40 CFR 711.15(b)).
- Manufacturers (including importers) are required to report processing and use data, for calendar year 2011, for all reportable chemical substances, when 2011 site-specific production volume equals or exceeds 100,000 lb (40 CFR 711.15(b)). Inorganic chemical substances are no longer exempt from the reporting of processing and use information.
- Small manufacturers are exempt from CDR requirements unless they manufacture (including import) 25,000 lb or more of a chemical substance that is the subject of a rule proposed or promulgated under sections 4, 5(b)(4), or 6 of TSCA, or is the subject of an order in effect under section 5(e) of TSCA, or is the subject of relief that has been granted under a civil action under sections 5 or 7 of TSCA (40 CFR 711.9) and (TSCA §8(a)(3)(A)(ii)). See Appendix B for further information.
- Information submitted under CDR may be claimed as confidential; however, such claims must be made at the time of submission and substantiated in accordance with the CDR rule. Submitters must provide upfront substantiation of confidentiality claims for processing and use information as well as for confidentiality claims for site or chemical identity. A blank response or a response that is designated as not known or reasonably ascertainable may not be claimed as confidential (40 CFR 711.30).
The EPA is frequently adding information to their Inventory Update Reporting and Chemical Data Reporting Resource page. And Emilcott will continue to update our TSCA Resource Center with helpful information -- check in often!
If you need guidance with the information that you should be gathering (starting now!) for the EPA TSCA 2012 CDR Submission, please contact Emilcott!.You can also subscribe to our TSCA newsletter to be kept up to date and enroll for our free webinar (date to be announced soon) by sending an email to pkaufmann@emilcott.com. Feel free to post any questions below in the comments section and we will respond quickly.
Topics: Emilcott, General EHS, EPA, Compliance, TSCA & R.E.A.C.H., TSCA, CDR, Toxic Substance Control Act, submission, IUR, Form U, chemical manufacturer, importer
Upcoming TSCA Reporting Period: February 1 - June 30, 2012.
As of August 6, the EPA has finalized the TSCA IUR -- now named the Chemical Data Reporting (CDR) Rule. There are many changes with the TSCA IUR to CDR some of these will be in place for the 2012 reporting submission and many more for the 2016 submission.
The final rule adopted many of the requirements included in the proposed rule (see What are the Changes?) -- and, thankfully, the majority are not retroactive. At Emilcott, we are asking our clients to define what needs to be collected for the 2012 submission period with 2011 as the Principal Reporting Year. If your facility uses chemicals or is an importer who falls under TSCAs CDR guidelines (remember, theyve changed!), your company will need to collect more data and information than that originally planned for the 2011 IUR submission. The chart below is a bare bones list of the inventory, production volume and use information that needs to be collected for the 2012 Chemical Data Report (CDR). And, as stated earlier, your 2016 submission will have even more requirements.
Submit Your Form U Electronically
For the 2012 CDF, all submissions will be required to use the EPAs free, web-based reporting tool, e-CDRweb, for completion of Form U. In preparation, the EPA will schedule another informational webinar on the electronic submission tool in late September with beta trials completed by early October. Please ask Emilcott if you will need help with the Form U filing.
Additional TSCA Information
Emilcott has set up an online TSCA Resource Center and over the next few weeks will be creating a dedicated TSCA landing page to contain all the information related to both the CDR Final Rule and other TSCA New and Existing Chemicals topics. Links to all EHSWire TSCA blogs will also be located there for quick reference. If you have any questions or would like to consider Emilcott as a TSCA consultant, please give us a call at 973-538-1110 or send an email to pkaufmann@emilcott.com
Did you miss the TSCA 2006 Form U submission?
You must notify the EPA that you missed reporting for the 2006 IUR within 21 days of your discovery. The EPA has an Audit Policy for Self-Disclosure in which drastic fine reduction is possible if the requirements outlined by the Agency are met. This policy is presented on the EPA Compliance Incentive and Auditing web site ( http://www.epa.gov/compliance/incentives/auditing/auditdisclose.html). If you would like help, Emilcott has been brought in to work with multiple US and International clients with US facilities that inadvertently missed the Form U deadline due to either misunderstanding or ignorance of the regulations. Our advice is to not wait!
Topics: Emilcott, General EHS, EPA, Compliance, TSCA & R.E.A.C.H., TSCA, CDR, Toxic Substance Control Act, submission, IUR, Form U, chemical manufacturer, importer
We have some news on this years TSCA IUR Form U submission. Well, it really isnt information about the requirements but we do know that this years Form U submission period will not be June 1 to September 30. It will most likely be later this year. So, we all can move that task to another segment of the calendar year!
More Information
On May 11, 2011, the EPA issued a Federal Register Notice amending the Toxic Substances Control Act (TSCA) Inventory Update Reporting (IUR) regulations by delaying the June 1 to September 30 submission period for the 2011 Form U reports. The notice indicated that this delay will not alter the timing of subsequent submission periods (e.g., the submission period from June 1, 2016 to September 30, 2016). This is an interesting statement as one of the changes included in the proposed IUR Rule is a change of the reporting period cycle to every four (4) years from the current five (5) year cycle.
The EPA is delaying the submission period because the proposed IUR modifications rule has not yet been finalized. EPA expects to have the final version of the changes to the IUR reporting requirements in the near future. The revised 2011 submission period will be announced with the publication of the final IUR modification rule.
How does this delay what the EPA rule refers to as a suspension affect what needs to be done for the 2010 reporting period? It seems that the EPA will mandate a new submission period but it is not clear when this will be during 2011.
- We are assuming that the reporting period will remain as the 2010 calendar year.
- Our next assumption, or guess, is that the Form U submission period will shift to September 1 to December 31, but that will require that the final rule on the IUR modifications be published very soon.
Food for Thought
As recently as March 4, 2011, representatives from the American Petroleum Institute (API) met with the EPA presenting concerns about several aspects of the proposed IUR modifications rule. One topic the API presented was that when the last set of revisions of the IUR was finalized in 2003 with the next reporting period was extended by one year shifting from 2004 to 2005 with Form U submission in 2006. During 2004 and 2005, the EPA held many workshops and issued clarification and guidance documents.
And, for now, we wait for the Final Rule and hope that the data we have all collected for the 2010 reporting period will be adequate. Emilcott's recommendations for what to do while we wait are in my January blog: TSCA IUR Update What Are the Changes ?. Essentially, we are advising our clients to proceed with the collection of 2010 inventory data with a threshold of 25,000 lbs. Here are a couple of items to keep on your radar:
- Be sure your list of manufactured chemicals is complete. Your list should be based on all chemical processes and imported materials received at the site and not just on the products.
- When calculating individual substance volumes include imported mixtures with those manufactured at the site aggregating all mixtures containing that substance.
...And, Emilcott will continue to keep you posted!
What to do if you need help or have questions?
If you need assistance related to the TSCA New Chemicals regulatory requirements or the potential changes due to the Inventory Update Reporting Rule, Emilcott can guide you through the reporting. We can also help you navigate the maze of reporting a potential Form U violation from prior filing years to the EPA (See http://www.emilcott.com/services/svcenvcompliance.asp).
As more information becomes available from the EPA regarding the IUR and as testing of the electronic tool begins, Emilcott will keep you up-to-date via EHSWire and our "Regulatory Updates" Newsletter.
Please give me a call at 1-800-886-3645 or write a comment below if you have any questions or additional information to contribute.
Topics: Emilcott, health and safety, General EHS, EPA, Compliance, TSCA & R.E.A.C.H., TSCA, Toxic Substance Control Act, reporting, regulation, chemicals
In the August 13, 2010 TSCA Inventory Update Reporting Modifications - Proposed Rule, the EPA anticipated the promulgation of the final rule by the Spring of 2011. Spring arrived 3 weeks ago, and the final IUR Reporting Modification rule has not been published. As such, the reporting modifications and specific reporting period have not been finalized. In addition, the Agency has not released a test version of the revised Form U electronic reporting software. It is possible that the Agency will change the proposed 2011 submission period (June 1 September 30) to another 4month period later in 2011.
EPA's intended final rule was sent to the White House Office of Management and Budget (OMB) on January 20, 2011. Since the beginning of February, OMB has held meetings with the American Chemistry Council, the Society of Chemical Manufacturers and Affiliates Inc. (SOCMA), the Small Business Administration, the National Mining Association, and associations that represent companies that must comply with the regulations.
So -- once again, the question is now what to do?
As mentioned in my January blog TSCA IUR Update What Are the Changes ? we are advising Emilcott clients to proceed with the collection of 2010 inventory data with a threshold of 25,000 lbs. Here are a couple of items to keep on your radar:
- Be sure your list of manufactured chemicals is complete. Your list should be based on all chemical processes and imported materials received at the site and not just on the products.
- When calculating individual substance volumes include imported mixtures with those manufactured at the site aggregating all mixtures containing that substance.
Additional data that may be needed for the 2011 reporting are listed below. Depending upon how you gather your information, you may want to request this along with the import or manufacturing volume information.
- Production volumes at or above 25,000 lbs directly exported and not domestically processed or used.
- All quantities of substances subject to rules and orders in the following sections:
- Section 5(a)(2) Significant New Use Rules (SNURs)
- Section 5(b)(4) Chemicals of concern to EPA
- Section 6 Prohibitions for chemicals with unreasonable risks
- Section 5(e) Requirements or restrictions on chemical production or use
- Section 5(f) Chemical with an unreasonable risk
What to do if you need help?
If you need assistance related to the TSCA New Chemicals regulatory requirements or the potential changes due to the Inventory Update Reporting Rule, Emilcott can guide you through the reporting. We can also help you navigate the maze of reporting a potential Form U violation from prior filing years to the EPA (See http://www.emilcott.com/services/svcenvcompliance.asp). As more information becomes available from the EPA regarding the IUR and as testing of the electronic tool begins, Emilcott will keep you up-to-date via EHSWire and our Regulatory Updates Newsletter. If you have any TSCA IUR questions or concerns, feel free to contact Emilcott or post your question below!
Topics: EPA, Compliance, TSCA & R.E.A.C.H., TSCA, Toxic Substance Control Act, reporting, regulation, chemicals