Environmental Health and Safety Blog | EHSWire
The 2012 TSCA CDR Submission Period Begins! Rev up your calculators and keyboards, NOW!!
Posted by Shivi Kakar
Topics: Emilcott, TSCA & R.E.A.C.H., TSCA, CDX registration, CDR, Toxic Substance Control Act
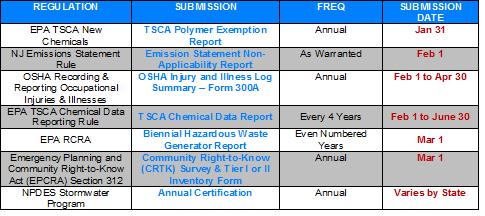
Regulatory Submissions & Postings Reminder (January thru April 2012)
Posted by Shivi Kakar
Topics: Emilcott, NPDES, OSHA, Emergency Planning and Community Right-to-Know Act, General Industry H&S, OSHA Compliance, General EHS, Construction H&S, EPA, EPCRA, Hazardous Waste Management, TSCA & R.E.A.C.H., TSCA, CDR, Toxic Substance Control Act, Biennial Hazardous Waste
Keep in Mind Polymers are EXEMPT from CDR Reporting!
Posted by Shivi Kakar
What polymeric substances are exempt from reporting?
- Chemical substances described with the word fragments *polym*, *alkyd, or oxylated in the Chemical Abstracts (CA) Index Name
- Chemical substance which is identified in the TSCA Master Inventory File as- Siloxane(s) and silicone(s)
- Silsesquioxane(s)
Topics: Emilcott, TSCA, CDR, CDR Reporting, polymeric substance, polymers, Uncategorized
What are the basic criteria needed to support a confidentially claim on the 2012 CDR Submission? Any business with a trade secret or confidential chemical formula is definitely wondering about this very issue as they consider just how to file their 2012 CDR Form U.
The EPA has increased the requirements (as in, made it more challenging) for companies who want to justify a claim of Confidential Business Information (CBI) in their CDR submission. A summary of the CDR CBI criteria for 2012, along with the long list of questions that must be addressed to support this claim are as follows (from 40 CFR 2.208):
- The business must show that disclosure of the information is likely to cause substantial harm to the business's competitive position.
- The confidentiality claim must be valid at the date of submission.
- The business can demonstrate that it has and still does protect the confidentiality of the information.
- The information is not (and hasnt been) obtainable by others without the business's consent.
- No statute specifically requires disclosure of the information.
What information can be claimed as Confidential Business Information (CBI) on the 2012 CDR Submission?
- Site
- Identity of the manufacturing or importing site linked with a reportable chemical substance. Site name, address, city, county, state, zip code, and Dun & Bradstreet number are protected.
- The company name must also be claimed as confidential to protect the link between the chemical identity and the company name.
- Chemical Substance
- Specific identity of a chemical substance (including Accession Number) -- only if EPA treats the identity of that chemical substance as confidential on the TSCA Inventory.
- Processing and Use Information
- Data associated with the processing and use information -- if you think this information would reveal trade secrets or confidential commercial or financial information.
What needs to be substantiated for the confidentiality claim to be accepted (honored) by the EPA?
All claims need to be substantiated at the time of submission. A blank response or a response that is designated as not known or reasonably ascertainable may not be claimed as confidential. There are specific questions for each type of claim as shown below -
Site
- Has site information been linked with a chemical identity in any other Federal, State or local reporting scheme? For example, is the chemical identity linked to a facility in a filing under the Emergency Planning and Community Right-to-Know ACT (EPCRA) section 311, namely through a Material Safety Data Sheets (MSDS)? If so, identify all such schemes. Was the linkage claimed as confidential in any of these instances?
- What harmful effect, if any, to your competitive position or to your customers competitive position do you think would result from disclosure of the processing and use data and the chemical substance? How could a competitor use such information? Would the effects of disclosure be substantial? What is the causal relationship between the disclosure and the harmful effects?
Chemical Identity the substantiation must be provided for each chemical substance claimed to be CBI
- What harmful effects to your competitive position, if any, or to your suppliers competitive position, do you think would result from the identity of the chemical substance being disclosed in connection with reporting under the CDR? How could a competitor use such information? Would the effects of disclosure be substantial? What is the causal relationship between the disclosure and the harmful effects?
- For how long should confidential treatment be given? Until a specific date, the occurrence of a specific event, or permanently? Why?
- Has the chemical substance been patented? If so, have you granted licenses to others with respect to the patent as it applies to the chemical substance? If the chemical substance has been patented, and therefore disclosed through the patent, why should it be treated as confidential?
- Has the identity of the chemical substance been kept confidential to the extent that your competitors do not know it is being manufactured or imported for a commercial purpose by anyone?
- Is the fact that the chemical substance is being manufactured (including imported) for a commercial purpose available to the public, for example, in technical journals, libraries, or State, local, or Federal agency public files?
- What measures have you taken to prevent undesired disclosure of the fact that the chemical substance is being manufactured (including imported) for a commercial purpose?
- To what extent has the fact that this chemical substance is manufactured (including imported) for commercial purposes been revealed to others? What precautions have been taken regarding these disclosures? Have there been public disclosures or disclosures to competitors?
- Does this particular chemical substance leave the site of manufacture (including import) in any form (e.g., as product, effluent, emission)? If so, what measures have been taken to guard against the discovery of its identity?
- If the chemical substance leaves the site in a product that is available to the public or your competitors can the chemical substance be identified by analysis of the product?
- For what purpose do you manufacture (including import) the chemical substance?
- Has EPA, another Federal agency, or any Federal court made any pertinent confidentiality determinations regarding this chemical substance? If so, please attach copies of such determinations.
Processing and Use Information - the substantiation must be provided for each process and use claimed to be CBI for a specific chemical substance
- Is the identified use of this chemical substance publicly known? Has your company ever provided use information on the chemical substance that was not claimed as confidential?
- What harmful effect, if any, to your competitive position or to your customers competitive position do you think would result from disclosure of the processing and use data and the chemical substance? How could a competitor use such information? Would the effects of disclosure be substantial? What is the causal relationship between the disclosure and the harmful effects?
- Obviously, manufacturers and other businesses who filed a CBI in 2006 must revisit their eligibility for their 2012 filing. And, to continue their eligibility, consideration for the extra time and effort to prepare the required substantiation documentation. To put it in a nutshell, with the number and depth of the EPAs questions that are involved, you better be prepared prior to making a CBI claim!
Did you file a CBI claim last year? Are you going to file a CBI claim for the same chemicals this year? If so, are you allowing for extra time (and budget!) to gather the substantiation information together? If not, why not?
Topics: EPA, TSCA & R.E.A.C.H., CDR, CBI, chemical substance, Confidential Business Information, 2012, submission
The EPA hosted a 3-hour webinar on November 16, 2011 that reviewed the reporting process for the 2012 Chemical Data Reporting (CDR) Rule with a focus on joint reporting, considerations related to the reporting of byproducts, and updated information about registering for electronic reporting and for using the electronic reporting tool/ The EPA has posted the presentation slides online and expects to have a recording of the webinar available for viewing by December 1.
Webinar Take Aways Still Many Questions
- The Agency has prepared a detailed instruction manual for the 2012 CDR that presents the reporting requirements using a decision logic diagram.
- Registration for the EPA Central Data Exchange (CDX) for CDR submission is scheduled to be opened on December 1. The sign-up link will be posted on the IUR/CDR Home Page. (Emilcott will also post it on our TSCA Resource Web Page.)
- e-CDR web, the CDR reporting tool, is scheduled to be available in January.
- The TSCA Substance Registry Services (SRS) will be updated in January.
- Additional resources will continue to be posted at www.epa.gov/cdr.
- A few of the new requirements highlighted during the presentation brought in many questions during the subsequent 2-hour webinar Q&A specifically concerning Contract Manufacturing, Joint Submission, and byproducts reporting.
Some of the questions not answered during the webinar that we found of particular interest are:
- What are the reporting responsibilities for toll manufacturers where the volume of a chemical made for one customer is less than 25,000 lbs but they manufacture for several customers putting the cumulative volume above the threshold?
- What does one do since the XML Schema that is currently posted does not function properly, and is stated to be the final version?
- Does starting material that is recycled (and reused) need to be reported since the material was not manufactured at the site?
- If off-specification material is reprocessed, does the material gained from the reprocessing get reported as a byproduct or is it included in the overall production volume?
- How does one account for non-isolated intermediates that are isolated and then reprocessed due to maintenance activities or upset conditions?
- What are the reporting responsibilities for an importer if the supplier does not agree to be a joint submitter?
As the reporting period nears we will be taking a careful look at the rationales and explanations provided in the Preamble to the Final Rule as the buck stops with the final rule as published.
If you need guidance for the EPA TSCA 2012 CDR Submission, Emilcott offers three helpful options
- Contact Emilcott directly with your questions about TSCA or other regulatory issues.
- Subscribe to our free TSCA newsletter which delivers TSCA-related information just like this right to your mailbox.
- Register for our free Dec 6 webinar: Do You Understand TSCA 2012 CDR Requirements?
Topics: EPA, TSCA & R.E.A.C.H., TSCA, CDR, 2012, submission, Preamble, Webinar, final rule, November 16
Emilcott TSCA Resource Center Expands with More Info and Options
Posted by Shivi Kakar
TSCA questions are pouring in and we are responding. To ensure that the information is available in a reasonable (and non-overwhelming) way, Emilcott has created a TSCA resource section of our web site for centralizing all kinds of intelligence, notifications, links, and summaries about Toxic Substance Control Act (TSCA) 2012 Chemical Data Reporting (CDR).
All these pages (just click on the headers) are available from the Emilcott Home page but we suggest you bookmark the pages that are most relevant and dont forget to register for our December 6th Free Webinar!
Emilcott TSCA Resource Center
The TSCA Resource Center has moved from the Emilcott home page to a new page lots of information including all our TSCA-related EHSwire blogs (CDR and IUR) and EPA Chemical Data Reporting Links, EPA New Chemicals Links, and EPA Import/Export Links. This page will stay updated so that you have a one-stop location for all TSCA information. If you have specific issues youd like to see addressed here, please let us know.
Topics: Compliance, TSCA & R.E.A.C.H., TSCA, CDR, Toxic Substance Control Act, IUR, violation, regulations, questions, filing, reporting, Chemical Data Reporting, Form U
Start collecting data for your 2012 TSCA Chemical Data Reporting submission now!
Posted by Shivi Kakar
We just had our first cold weather snap in the northeast reminding me that 2012 is just around the corner. A change in the seasons is our cue to ask Emilcott clients that manufacture or import chemicals: Have you gathered the 2010 chemical volume data AND are you collecting the 2011 data needed for the 2012 TSCA Chemical Data Report?
In a previous blog we summarized the basic requirements of the inventory, production volume and use i nformation that needs to be collected for the EPAs TSCA 2012 Chemical Data Report (CDR). You can find this bare bones chart by clicking here. The EPA recently presented the following key requirements in this document Instructions for the 2012 TSCA Chemical Data Reporting.
HIGHLIGHTS OF 2012 TSCA CHEMICAL DATA REPORTING (CDR)
- The determination of the need to report is based on production volume during calendar year 2011.
- Information on the reportable chemical substance must be reported during the 2012 CDR submission period, February 1, 2012 to June 30, 2012 (40 CFR 711.20).
- All reporting companies must report CDR data electronically, using e-CDRweb, the CDR web-based reporting tool, and EPAs Central Data Exchange (CDX) system. Prior to submitting data, submitters must register with CDX.
- Reporting is required for all chemical substances listed on the TSCA Inventory, both organic and inorganic, other than polymers, microorganisms, naturally occurring chemical substances, certain forms of natural gas, and water (40 CFR 711.5 and 711.6) when manufacture (including import) of those chemical substances meets the other reporting requirements. Chemical substances that are the subject of any of certain listed TSCA actions may not be eligible for partial or full exemptions (40 CFR 711.6).
- Manufacturers (including importers) are required to report full manufacturing data, for calendar year 2011, and production volume only, for calendar year 2010, for all reportable chemical substances, when 2011 site-specific production volume equals or exceeds 25,000 lb (40 CFR 711.15(b)).
- Manufacturers (including importers) are required to report processing and use data, for calendar year 2011, for all reportable chemical substances, when 2011 site-specific production volume equals or exceeds 100,000 lb (40 CFR 711.15(b)). Inorganic chemical substances are no longer exempt from the reporting of processing and use information.
- Small manufacturers are exempt from CDR requirements unless they manufacture (including import) 25,000 lb or more of a chemical substance that is the subject of a rule proposed or promulgated under sections 4, 5(b)(4), or 6 of TSCA, or is the subject of an order in effect under section 5(e) of TSCA, or is the subject of relief that has been granted under a civil action under sections 5 or 7 of TSCA (40 CFR 711.9) and (TSCA §8(a)(3)(A)(ii)). See Appendix B for further information.
- Information submitted under CDR may be claimed as confidential; however, such claims must be made at the time of submission and substantiated in accordance with the CDR rule. Submitters must provide upfront substantiation of confidentiality claims for processing and use information as well as for confidentiality claims for site or chemical identity. A blank response or a response that is designated as not known or reasonably ascertainable may not be claimed as confidential (40 CFR 711.30).
The EPA is frequently adding information to their Inventory Update Reporting and Chemical Data Reporting Resource page. And Emilcott will continue to update our TSCA Resource Center with helpful information -- check in often!
If you need guidance with the information that you should be gathering (starting now!) for the EPA TSCA 2012 CDR Submission, please contact Emilcott!.You can also subscribe to our TSCA newsletter to be kept up to date and enroll for our free webinar (date to be announced soon) by sending an email to pkaufmann@emilcott.com. Feel free to post any questions below in the comments section and we will respond quickly.
Topics: Emilcott, General EHS, EPA, Compliance, TSCA & R.E.A.C.H., TSCA, CDR, Toxic Substance Control Act, submission, IUR, Form U, chemical manufacturer, importer
Upcoming TSCA Reporting Period: February 1 - June 30, 2012.
As of August 6, the EPA has finalized the TSCA IUR -- now named the Chemical Data Reporting (CDR) Rule. There are many changes with the TSCA IUR to CDR some of these will be in place for the 2012 reporting submission and many more for the 2016 submission.
The final rule adopted many of the requirements included in the proposed rule (see What are the Changes?) -- and, thankfully, the majority are not retroactive. At Emilcott, we are asking our clients to define what needs to be collected for the 2012 submission period with 2011 as the Principal Reporting Year. If your facility uses chemicals or is an importer who falls under TSCAs CDR guidelines (remember, theyve changed!), your company will need to collect more data and information than that originally planned for the 2011 IUR submission. The chart below is a bare bones list of the inventory, production volume and use information that needs to be collected for the 2012 Chemical Data Report (CDR). And, as stated earlier, your 2016 submission will have even more requirements.
Submit Your Form U Electronically
For the 2012 CDF, all submissions will be required to use the EPAs free, web-based reporting tool, e-CDRweb, for completion of Form U. In preparation, the EPA will schedule another informational webinar on the electronic submission tool in late September with beta trials completed by early October. Please ask Emilcott if you will need help with the Form U filing.
Additional TSCA Information
Emilcott has set up an online TSCA Resource Center and over the next few weeks will be creating a dedicated TSCA landing page to contain all the information related to both the CDR Final Rule and other TSCA New and Existing Chemicals topics. Links to all EHSWire TSCA blogs will also be located there for quick reference. If you have any questions or would like to consider Emilcott as a TSCA consultant, please give us a call at 973-538-1110 or send an email to pkaufmann@emilcott.com
Did you miss the TSCA 2006 Form U submission?
You must notify the EPA that you missed reporting for the 2006 IUR within 21 days of your discovery. The EPA has an Audit Policy for Self-Disclosure in which drastic fine reduction is possible if the requirements outlined by the Agency are met. This policy is presented on the EPA Compliance Incentive and Auditing web site ( http://www.epa.gov/compliance/incentives/auditing/auditdisclose.html). If you would like help, Emilcott has been brought in to work with multiple US and International clients with US facilities that inadvertently missed the Form U deadline due to either misunderstanding or ignorance of the regulations. Our advice is to not wait!
Topics: Emilcott, General EHS, EPA, Compliance, TSCA & R.E.A.C.H., TSCA, CDR, Toxic Substance Control Act, submission, IUR, Form U, chemical manufacturer, importer
BREAKING NEWS: New EPA TSCA Inventory Update Requirements (IUR) for 2012
Posted by Shivi Kakar
Highlights That You Should Know
- The 2012 Submission Period is scheduled to occur February 1, 2012 - June 30, 2012.
- Production (manufacture or import) volumes are required for 2010 and 2011--- with manufacturing, processing and use information from 2011.
- TSCA Form U impacts all chemical manufacturers and importers and certain chemical users and processors.
- IUR is now called CDR Chemical Data Reporting Rule.
- The CDR Rule includes significant changes.
Reporting thresholds are for (a) substances in cumulative volumes of 25,000 lbs or more in the specific reporting year (2010, 2011) and (b) p rocessing and use information for substances manufactured (including imported) 100,000 lbs (not 300,000 lbs) or more in 2011.
How to Stay Up-to-Date with Incoming TSCA Information*
Now that EPA has scheduled the submission period and defined what has to be reported, and how it must be done, Emilcotts EHS group is compiling a centralized, online resource to help our clients complete the reporting process accurately and on time. As they become available, these materials will be emailed to Emilcott TSCA News Update subscribers or upon request:
- A comprehensive summary of the revised requirements and critical changes to the 2012 Form U submission (available upon request within a week).
- Notification of EPA seminars and other TSCA-related information activities. Emilcott will create updates for these sessions as they occur.
- Free informational TSCA-related CDR Rule Form U webinars.
Visit our TSCA Resource Center on the Emilcott home page frequently for EPA docs and other TSCA links.
*Continued technical information, updates and webinar invitations will be sent to subscribers of Emilcott's TSCA News Update. To subscribe or find out more information, call 800-886-3645 or send an email to pkaufmann (at) emilcott.com.
Additional Questions?
If you need any assistance related to the TSCA New Chemicals regulatory requirements or the potential changes due to the IUR/CDR Rule, Emilcott can guide you through the reporting process. Have questions about your 2006 TSCA Submission (or lack of)? We can also help you navigate the maze of reporting a potential Form U violation from prior filing years to the EPA. If you are gearing up for TSCA information gathering, subscribe to the Emilcott TSCA News Update, check the Emilcott TSCA Resource Center often, and, of course, stay tuned and subscribe to EHSWire!
Topics: EPA, TSCA & R.E.A.C.H., TSCA, CDR, submission, IUR, Form U